In response to the need for better batteries, Asahi Kasei conceived and developed the first Lithium-ion (Li-ion) battery. The technology was then first commercialized by Sony, and followed by A&T Battery Co. (a joint company of Toshiba Battery and Asahi Kasei Co.) in 1992.
Lithium-ion technology was immediately accepted because of its high-energy density, greater performance, and no memory effect as occurred, if compared to the previous Nickel-cadmium (Ni–Cd) or nickel-hydride (Ni–MH) batteries.
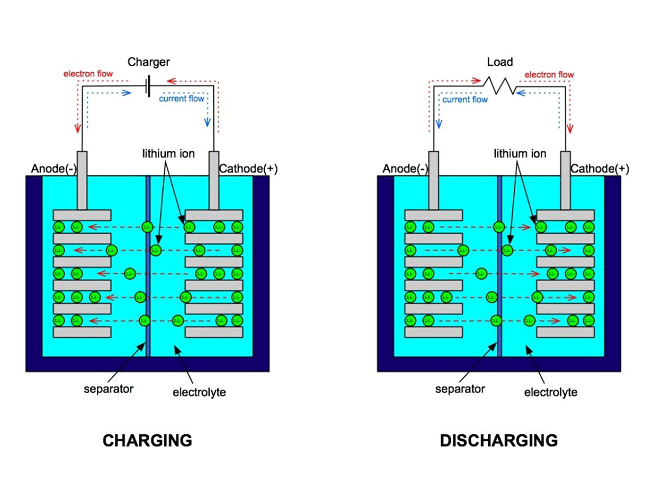
There are three primary functional components inside a Li-ion battery are the positive and negative electrodes and electrolyte.
Generally, the negative electrode of a Li-ion cell is made from carbon or graphite, while the positive electrode is made from layered oxide (such as lithium cobalt oxide, lithium iron phosphate or lithium manganese oxide), and the electrolyte is a lithium salt in an organic solvent (a mixture of organic carbonates such as ethylene carbonate or diethyl carbonate containing complexes of lithium ions).
Depending on which materials are used, the voltage, energy density, life, and safety of a lithium-ion battery can change.
While Li-ion batteries are more capable than Ni–Cd, they are more fragile, has safety hazards since they contain a flammable electrolyte and may be kept pressurized, and require protection circuits. But still, they are efficient.
This makes them common in home electronics, and popular for portable electronics, with a high energy density, tiny memory effect and low self-discharge.
